Pluri and Bar-Ilan University to Develop PLX Cells for the Treatment of Cocaine Addiction
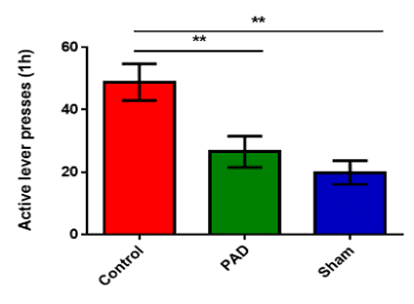
- Agreement signed following positive pre-clinical data demonstrating a one-time treatment with PLX-PAD significantly increased neurogenesis, offering immediate, long-term therapeutic effect in treating cocaine addiction
- There are no FDA-approved medications to treat cocaine addiction, a severely unmet need as demand for treatment increases in the U.S. and Europe
- Bar-Ilan University through its tech transfer arm BIRAD Research & Development Company Ltd. receives the right to develop and commercialize the product, Pluri entitled to 20% revenue sharing from future sales of PLX cells as anti-addiction product
HAIFA, Israel, December 21, 2023 – Pluri Inc. (Nasdaq: PLUR) (TASE: PLUR) (“Pluri” or the “Company”), a leading biotech company that transforms cells into solutions that promote well-being and sustainability, announced it has signed an agreement assigning the joint patent rights to develop Pluri’s PLX cells in the treatment of cocaine addiction, to BIRAD–Research & Development Company Ltd., the commercial arm of Bar-Ilan University. Under the agreement, Bar-Ilan University via BIRAD will receive the right to further develop and commercialize PLX cells as a cocaine anti-addiction product, and Pluri is entitled to 20% revenue sharing from future sales of the product for anti-addiction.
The agreement stems from a collaboration between Pluri and Bar-Ilan University researchers that presented compelling findings. Data from the studies were published in the peer–reviewed journal Pharmaceutics.
the press release:

Recent Comments